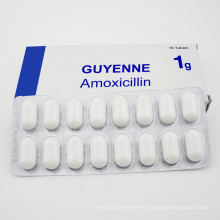
Amopicillin Ampiclox y Amoxici Tablet Llin
Información básica
Modelo: Am-160629AMXL
Descripción del producto
Modelo NO .: Am-160629AMXL Modo de uso: Para administración oral Estado: sólido Tipo: Productos biológicos Marca: Guyena Origen: Francia Aplicación: Medicina interna Adecuado para: todos Forma: tableta Tecnología farmacéutica: Extracción de producto natural Especificación: 250 mg / 500 mg Código HS : 2941109200 \ n 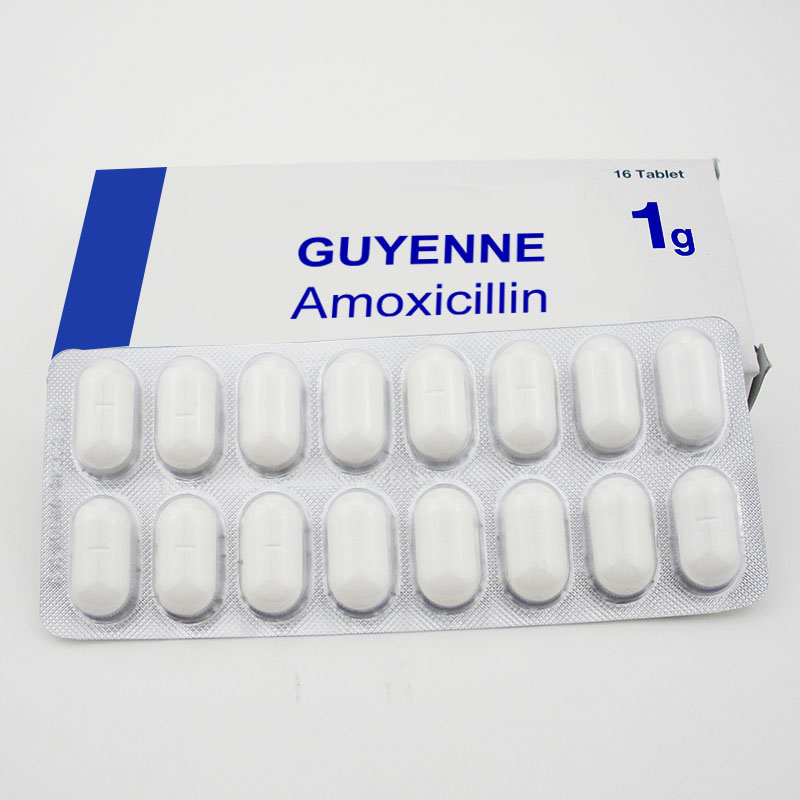 \ nProduct Description \ nprueba de pacientes positivos alérgicos a la penicilina y la penicilina en la piel con discapacidad
\ nProduct Description \ nprueba de pacientes positivos alérgicos a la penicilina y la penicilina en la piel con discapacidad
 \ nProduct Description \ nprueba de pacientes positivos alérgicos a la penicilina y la penicilina en la piel con discapacidad
\ nProduct Description \ nprueba de pacientes positivos alérgicos a la penicilina y la penicilina en la piel con discapacidad | Product: | tablet |
| Indications | Otitis media, sinusitis, pharyngitis, tonsillitis, urinary tract infections, skin and soft tissue infections, acute bronchitis, pneumonia, gonorrhea, typhoid, infectious diseases, sexually transmitted diseases, dermatology, renal medicine, respiratory medicine, ENT, purulent pleurisy, hepatobiliary system infections, sepsis, dysentery. |
| Dosage | Oral. 1, adults once 0.5g, every 6 to 8 hours a day dose does not exceed 4g. 2, children daily dose according to body weight 20 ~ 40mg / kg, every 8 hours; three months following the baby daily dose according to the weight 30mg / kg, every 12 hours. 3, patients with severe renal impairment need to adjust the dose, which endogenous creatinine clearance rate of 10 ~ 30ml / min in patients with 0.25 ~ 0.5g every 12 hours; patients with creatinine clearance less than 10ml / min 0.25 per 24 hours ~ 0.5g. |
| Adverse reactions | 1. Nausea, vomiting, diarrhea and pseudomembranous colitis and other gastrointestinal reactions. 2. Rash, drug fever and asthma and other allergic reactions. 3. Anemia, thrombocytopenia, eosinophilia cells. 4. Serum aminotransferase may be slightly increased. 5. Caused by the Candida or resistant superinfection. 6. Occasionally excitement, anxiety, insomnia, dizziness, and behavioral abnormalities, central nervous system symptoms. |
| Taboo | test-positive patients allergic to penicillin and penicillin skin disabled. |
| NOTES | 1. It must be done before using sodium penicillin skin test positive for the banned. 2. Infectious mononucleosis in patients with the FDA should be prone to rash, should be avoided. 3. Longer course of treatment patients should check liver and kidney function and blood. 4. It can lead to the use of Benedict or Fehling reagent false positive urine test. 5. It should be used with caution in the following cases: (1) have asthma, a history of hay fever and other allergic diseases. (2) may have to adjust the dose elderly and severe renal impairment |
Grupos de Producto : Antibióticos
Premium Related Products
Otros productos
Productos hot
Comprimidos de clorhidrato de amiodarona de alta calidad 200mgCrema de Monobenzona de Piel Blanqueadora GrandeMedicina General Omeprazol 20mg Inyección para Gastrohelcosis y Acido EstomacalMedicina General Ceftriaxone Sodium InjectionBlanqueamiento de la piel lista de productos anti-envejecimiento de la inyección de vitamina COEM Tablet 500mg ParacetamolCápsulas duras de la alta calidad 500mg Amoxicillin (amoxicilina)Mejor y bajo precio ceftriaxona sodio inyección ceftriaxonaMejor calidad Omega 3 cápsula de mar profundo Cápsula aceite de pescadoArtemisinina Antimalárica Curativa Aprobada por la FDATratamiento de lesiones cerebrales Citicoline Sodium InjectionProteasa Alfa Quimolasa para Edema InflamatorioMedicina general Inyección de acetato de medroxiprogesteronaAmikacin Inyecciones Medicina General MedicamentosBlanqueamiento de la piel Gsh Personal Care Inyección de glutatiónBody Slimming Fitness Perder Peso Pérdida de Peso L-Carnitine Injection2.0g / 5ml